Mass Defect and Binding Energy
Mass Defect and Binding Energy: Overview
This topic covers concepts, such as Pair Production, Pair Annihilation, Mass Defect, Binding Energy, Binding Energy per Nucleon, Dependence of Nuclear Stability on Binding Energy per Nucleon, Mass Spectrometer, etc.
Important Questions on Mass Defect and Binding Energy
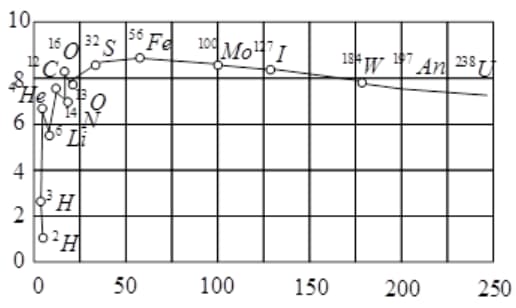
The plot of the binding energy per nucleon versus the mass number A for a large number of nuclei, is shown in Fig. In which range the binding energy per nucleon is constant?
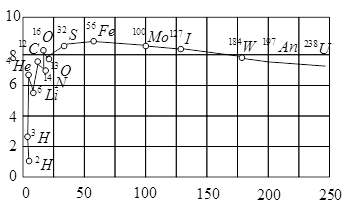
The plot of the binding energy per nucleon versus the mass number A for a large number of nuclei, is shown in Fig. In which range the binding energy per nucleon is constant?
Calculate the energy released in MeV in the following nuclear reaction :<
[Mass of = 238.05079 u]
Mass of = 234.043630 u
Mass of = 4.002600 u
1 u = 931.5 MeV
Calculate the binding energy per nucleon of nucleus.
Given:
Mass of ,
Mass of proton ,
and
The value of binding energy per nucleon of nucleus is
Given:
Mass of nucleus
Mass of proton
Mass of neutron
and
The binding energies per nucleon for deuteron () and helium () are and respectively. The energy released when two deuterons fuse to form a helium nucleus () is
In the nuclear process, stands for _______
In the following, column I lists some physical quantities and the column II gives approximate energy values associated with those. Choose appropriate values of energies as per the choices given below
| Column I | Column II | ||
| Energy of thermal neutrons | |||
| Binding energy per nucleon | |||
| Energy of X-rays | |||
| Photoelectric threshold of a meta | |||
The above is a plot of binding energy per nucleon against the nuclear mass correspond to different nuclei. Consider four reactions :
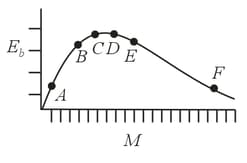
(i)
(ii)
(iii) and
(iv)
where is the energy released. In which reactions positive
Four hydrogen nuclei combine to form a helium nucleus. If the mass defect in the formation of helium is and one of hydrogen undergoes fusion to form helium, the energy released in kilowatt hour is
Two deuterons undergo fusion to form a helium nucleus as . Given binding energy per nucleon for and are and , respectively. The -value is
The difference between the mass of a nucleus and the sum of the masses of the individual nucleons is Which of the following is approximately the binding energy of the nucleus?
A star consist of deuterons. It initially has deutrons. It produces energy by the processes
If the average power radiated by the star is watt and masses of nuclei are
and use approximation,
energy equivalent to . Find the time in which the supply of deuteron in the star is exhausted.
The mass of deuteron nucleus is If the masses of proton and neutron are and respectively, calculate the binding energy per nucleon.
Binding energy of a nucleus is,
The binding energy per nucleon is maximum in the case of,
Obtain the binding energy of a nitrogen nucleus Given mass of proton mass of neutron
One requires energy to remove a nucleon from a nucleus and an energy to remove an electron from the orbit of an atom then :-
If energy released per fission is , then the number of fission reactions of per second for the production of power will be,
Which of the following nucleus having highest binding energy per nucleon:
